
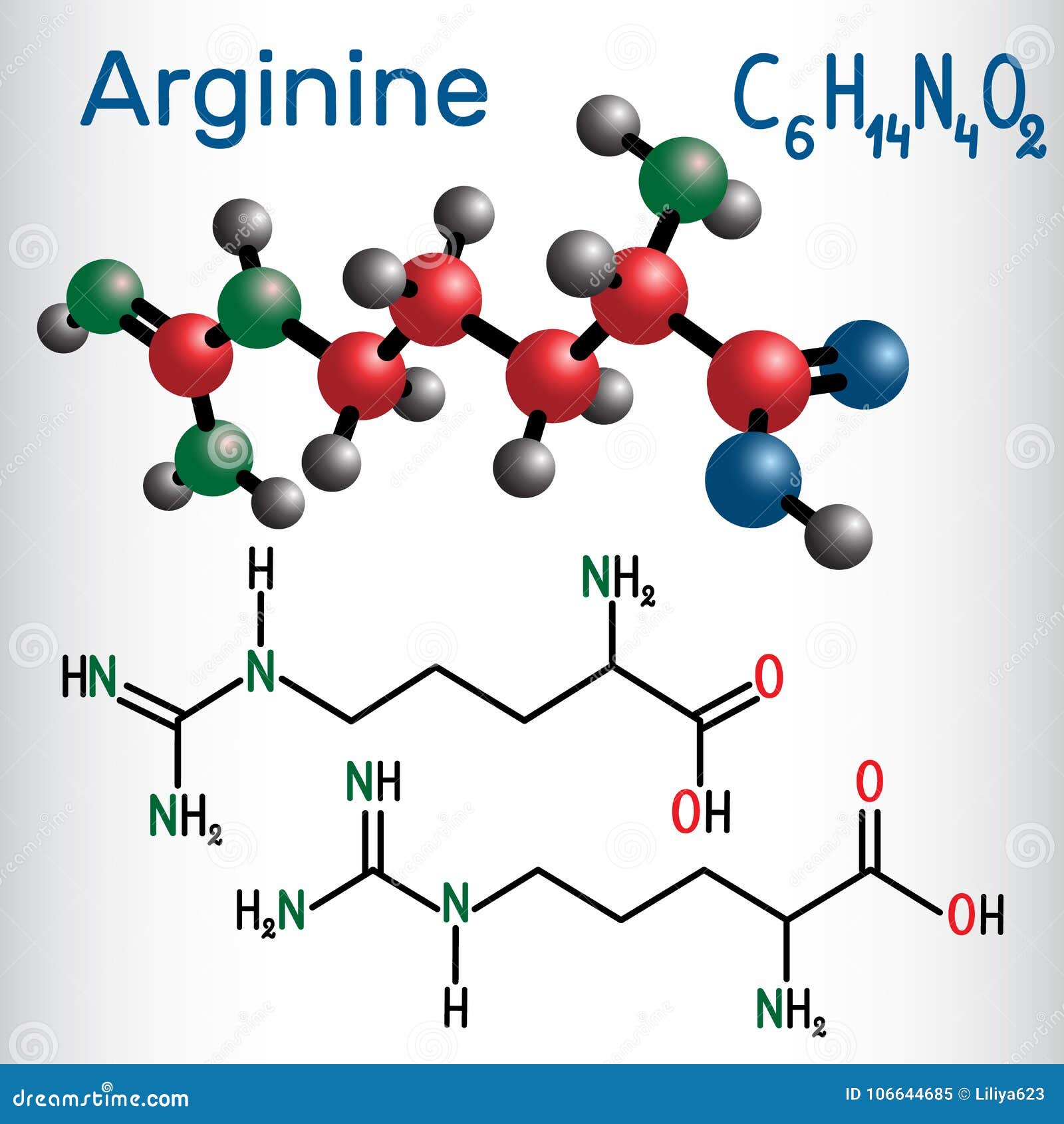
Only carbon and hydrogen in their side chains.The most helpful start-point is to separate amino acids into: Nonpolar Neutral polar Charged polar 1. HA H+ + A- Ka = Ka x -log = -log Ka -log pH = pKa + log Henderson/Hasselbach equation and pKa Protonated form Unprotonated form (conjugate base)Ĭlassification of Amino Acids Amino acids are generally divided into groups on the basis of their side chains (R groups). Proton donor Proton acceptor Fully protonated form at wery low pH Izoelectric point Dipolar ion At the midpoint – pK1=2.34 there is equimolar concentration of proton donor and proton acceptor.
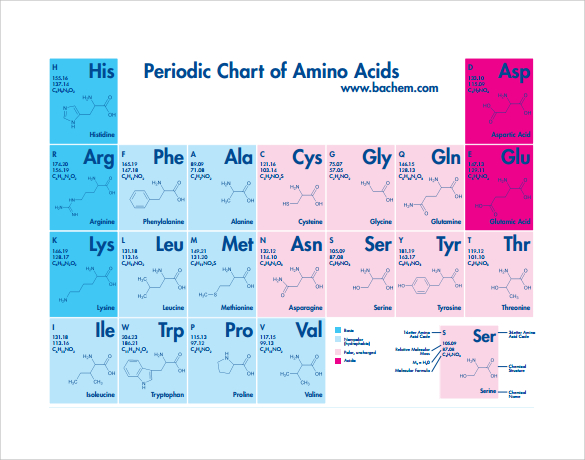
+ + Amino acids have characteristic titration curves Proton donor Proton acceptor At the midpoint – pK=9.60 there is equimolar concentration of proton donor and proton acceptor. Nonionic and zwitterion forms of amino acids The zwitterion predominates at neutral pH Zwitterion = in German for „hybrid ion“ Week acid Week baseĪ simple monoamino monocarboxyl a-amino acid is a diprotic acid (can yield protons) when fully protonated L-isomer L-isomer is normally found in proteins. The basic structure of amino acids differ only in the structure of the or the side chain (R-group). Several genetic disorders are cause in amino acid metabolism errors (aminoaciduria - presence of amino acids in urine).They can have hormonal and catalytic function.They are important intermediates in metabolism (porphyrins, purines, pyrimidines, creatin, urea etc).Their chemical structure influences three dimensional structure of proteins.Their molecules containing both amino and carboxyl groups attached to the same a-carbon (L-a-amino acids).Proteins are composed of 20 different amino acid (encoded by standard genetic code, construct proteins in all species ).Amino acids are building blocks of proteins.


 0 kommentar(er)
0 kommentar(er)
